
High shear mixer emulsification High shear mixer emulsification Additionally, it enables the particles to stay well mixed for longer. A substance’s particles can readily and easily join with the particles of another substance by having smaller particles. The advantages include increased product viscosity, shelf life, homogeneity, consistency, stability, flavor, and color. Homogenization is the process of spreading solid particles evenly throughout a liquid or emulsifying two immiscible liquids.
#Water in oil cream example how to#
Ice cream: oil and air in water emulsionĮmulsion solution: How to fix a broken emulsion.Crema on espresso: an emulsion of water and coffee oil.Mayonnaise: an emulsion of oil in water.Egg yolk: contains the emulsifying agent lecithin.Sorbitan stearate, polyglyceryl oleate, lecithin, sorbitan monooleate, and lanolin are frequently used without emulsifiers. These emulsions use water as the dispersion medium while o/w emulsifiers keep oil droplets condensed in the liquid.Įmulsification in food processing is w/o particularly helpful in goods made for dry or sensitive skin, such as butter, margarine, cold cream, and cod liver oil. They absorb water, are non-greasy, non-occlusive, and can be mixed with water. The emulsification process in cosmetics is o/w emulsions are used in moisturizing cosmetics and food items like milk, mayonnaise, and vinaigrette because they have a low oil concentration. Emulsification of oil and water: 5 Common examples This is what is the significance of emulsification of fat because digestion and absorption of lipids depend on emulsification. Pancreatic lipase breaks down these emulsified fats into fatty acids and glycerol.

The smaller globules of fat are broken down by bile salts, creating a milky emulsion. Process of emulsification: how does emulsification work

Salts are then secreted and kept in the gallbladder. In digestion, the small intestine is where emulsification takes place by breaking it down into smaller fat globules. It can be created mechanically (using a high-energy) or chemically (low-energy method).
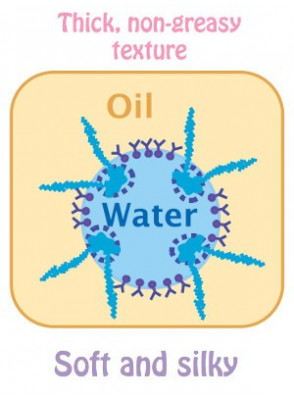
Image Source: Emulsifier for oil and water mixtureĭispersing one liquid into another immiscible liquid is known as emulsification. Can make oil and water mix for prolonged lengths of time. That mixing, though, ends quickly! After a few minutes, you can recheck the container to see that the water and oil have separated. Have you shaken water and oil before? If so, you are aware that they did mix briefly. Water and oil don’t mix when you add one to the other.

It is because water is a polar molecule, meaning that one end of it is negatively charged, the other positively charged. Image Source: Do oil and water mix together. The polar chemistry of the base stock and oil additions affects the dew point temperature. Condensation causes fat to become cloudier as it gets cooler. When oil reaches its dew point, it has a 100% concentration of dissolved water. Image Source: Does oil have water in it?


 0 kommentar(er)
0 kommentar(er)
